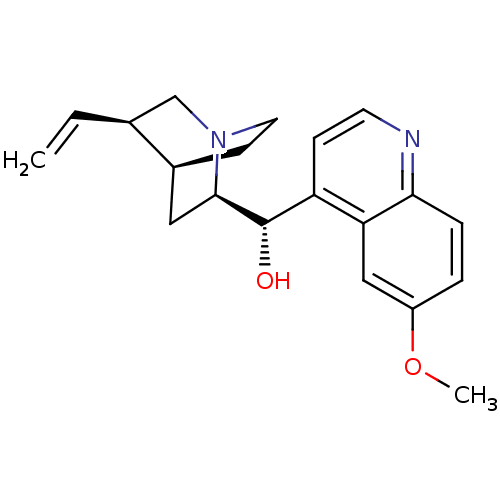
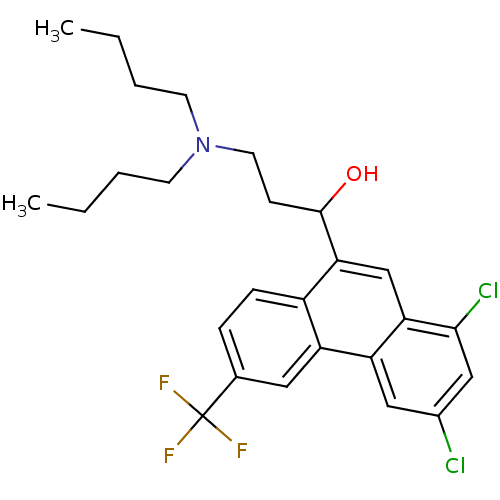
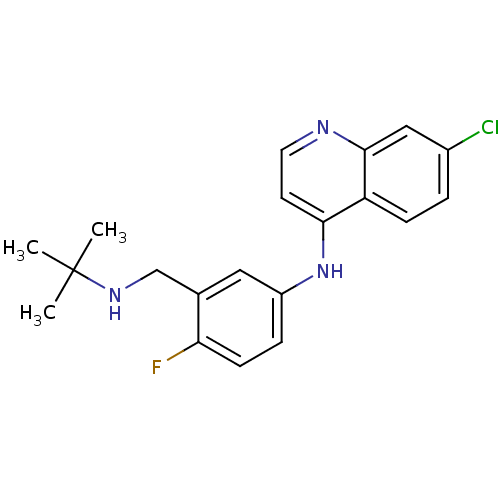
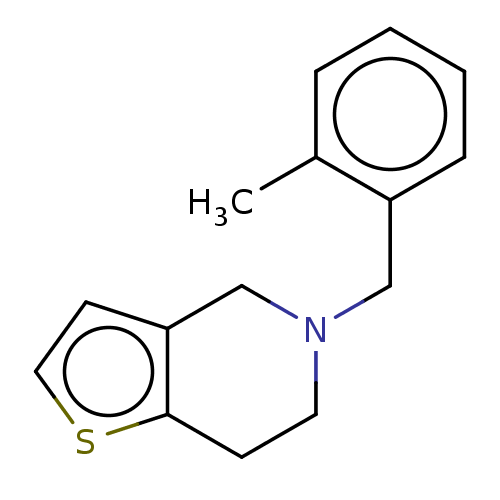
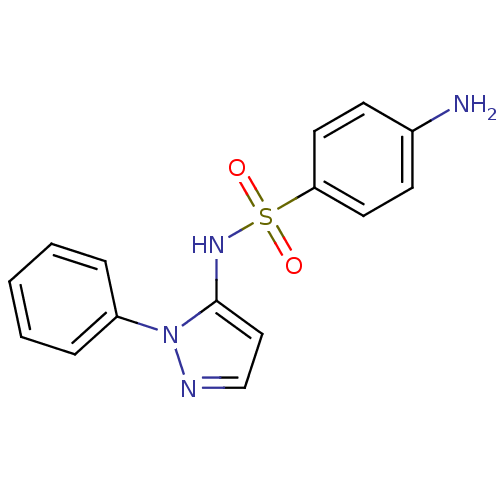
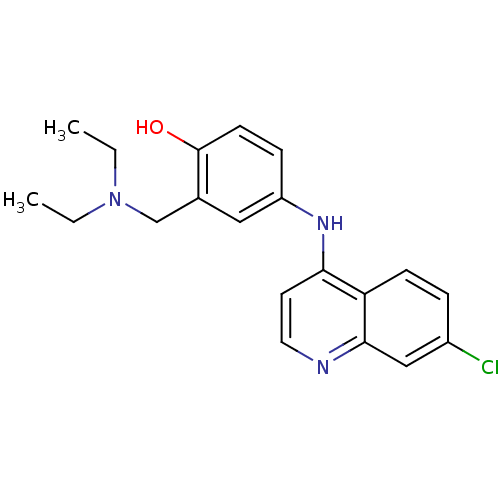
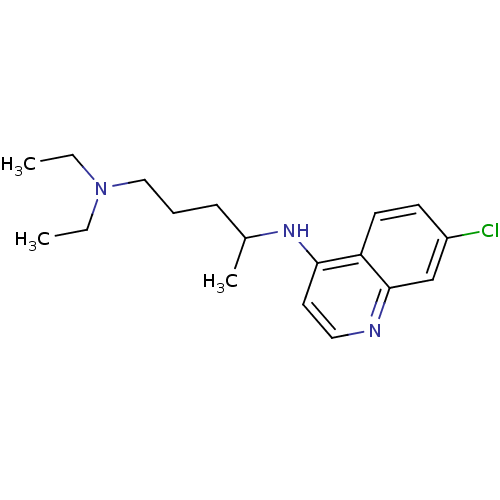
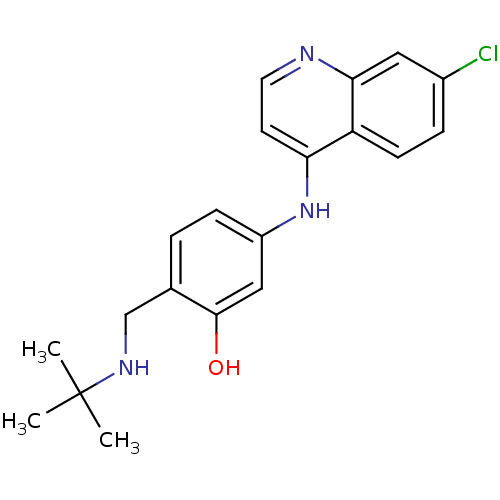
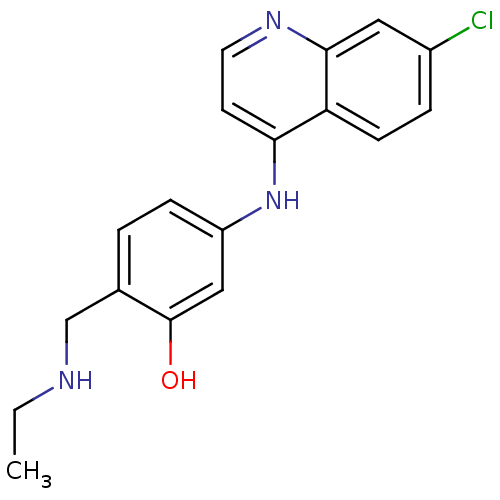
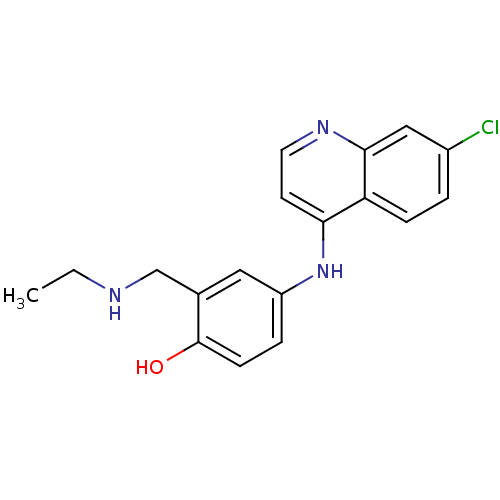
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity in absence of detergentMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 99nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 900nM ΔG°: -8.57kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 950nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nM ΔG°: -8.35kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nM ΔG°: -8.00kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 3.80E+3nM ΔG°: -7.69kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 4.90E+3nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 5.40E+3nM ΔG°: -7.47kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity in absence of detergentMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 9.10E+3nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 1.92E+4nM ΔG°: -6.69kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+4nM ΔG°: -6.66kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+4nM ΔG°: -6.16kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+4nM ΔG°: -6.03kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase D(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataKi: 6.30E+4nMAssay Description:Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based...More data for this Ligand-Target Pair
Affinity DataKi: 1.37E+5nM ΔG°: -5.48kcal/molepH: 7.0 T: 2°CAssay Description:AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human recombinant CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as phenacetin O-deethylation after 4 to 40 mins in presence of NADPH by LCMS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human recombinant CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University Of Liverpool
Curated by ChEMBL
University Of Liverpool
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of human cloned ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human recombinant CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human recombinant CYP1A2More data for this Ligand-Target Pair

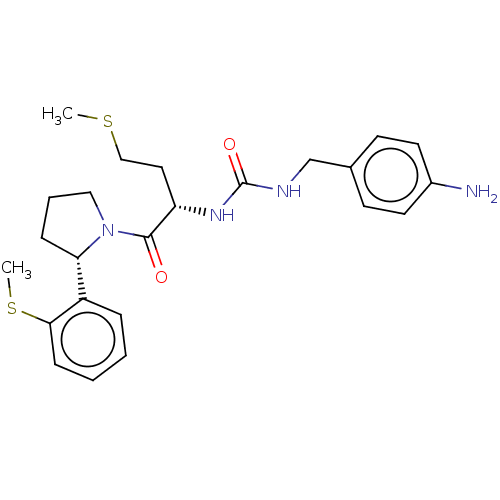
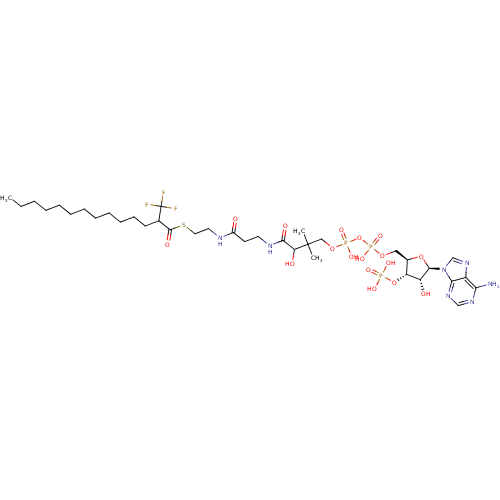
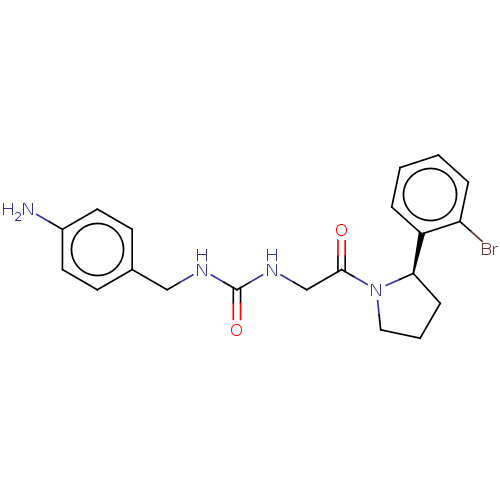
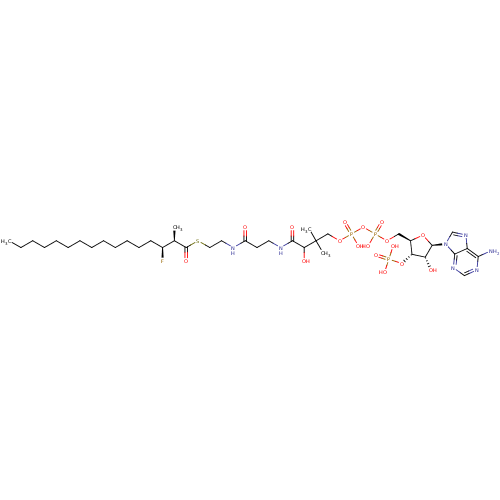
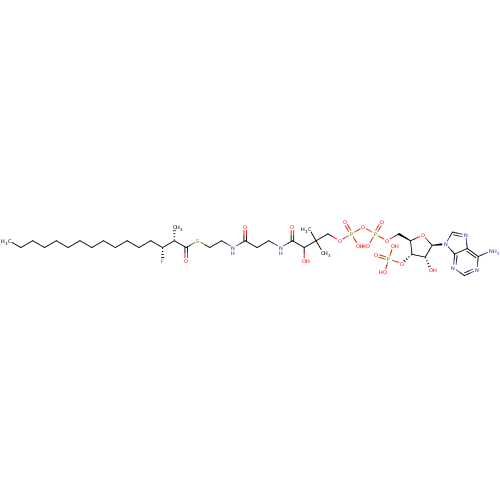
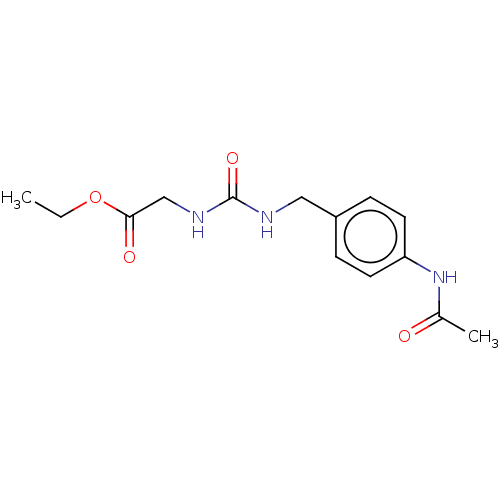
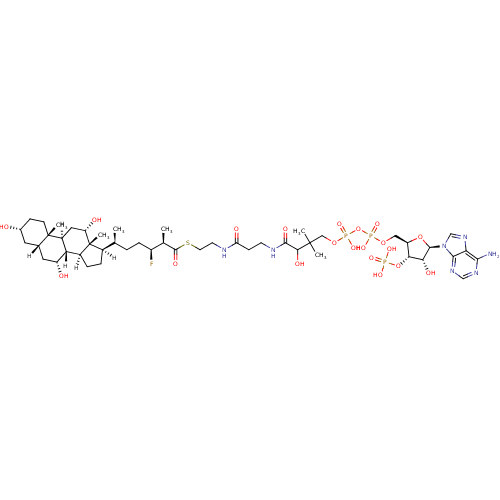
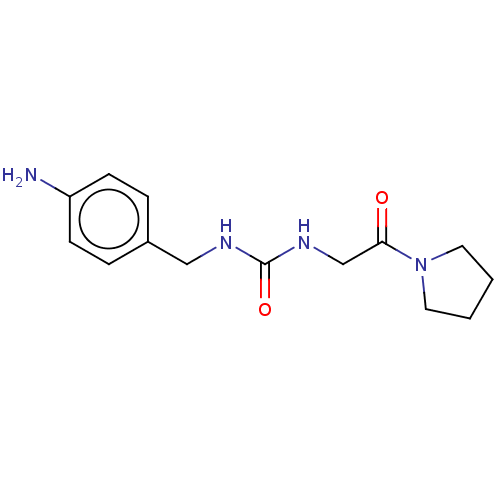
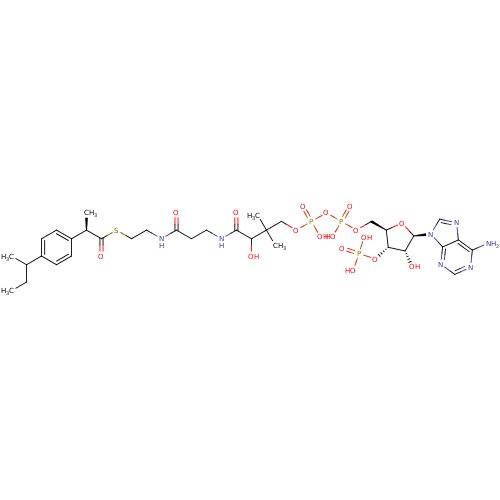
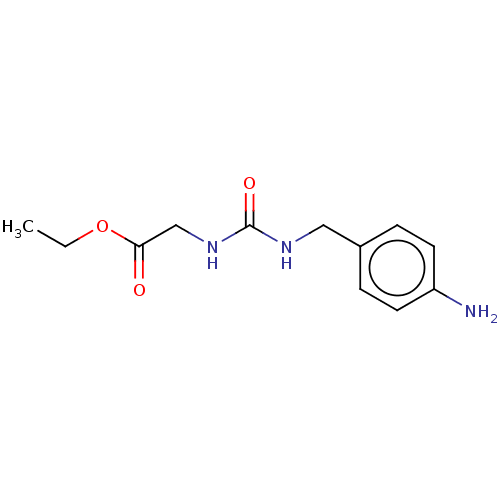
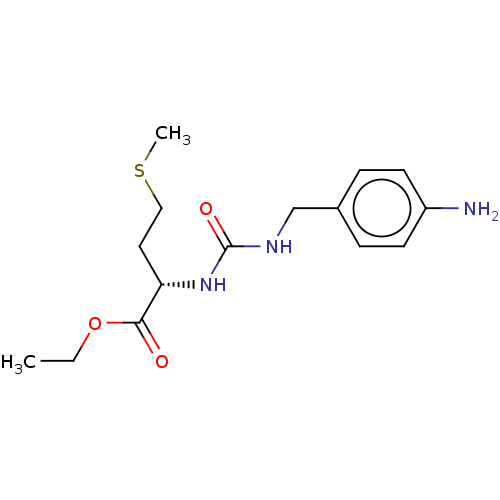
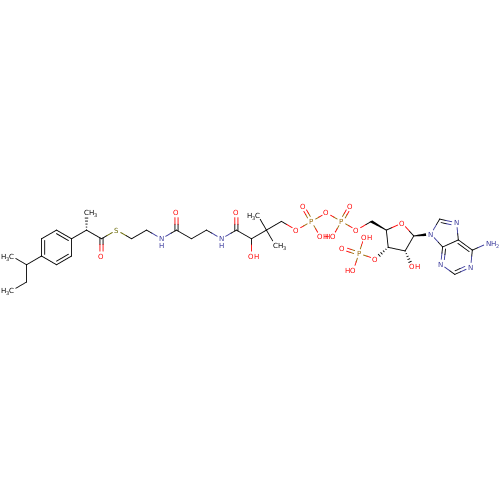
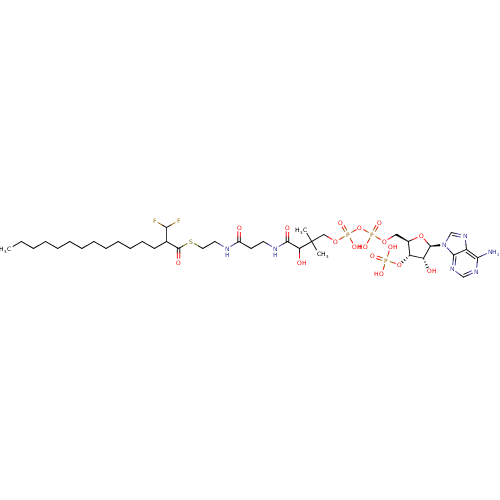
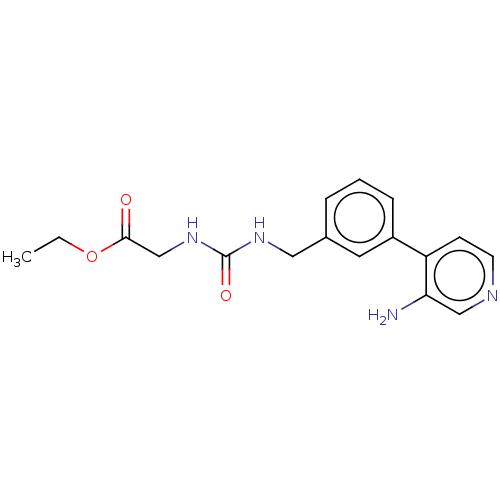

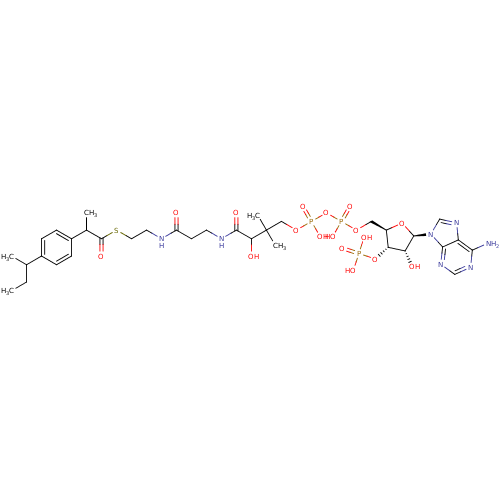
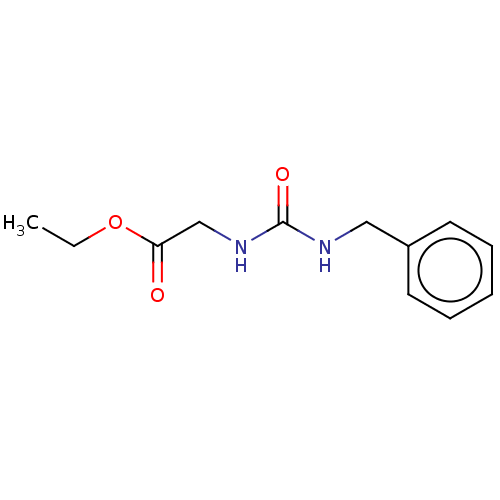

 3D Structure (crystal)
3D Structure (crystal)